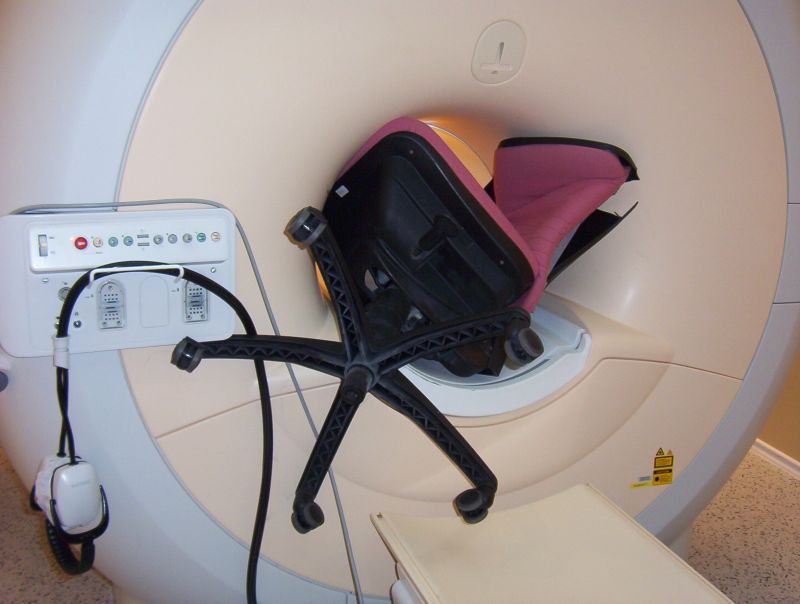
An office chair was in the wrong place - at ANY time!

MRI TESTING OF MEDICAL IMPLANTS, MATERIALS, AND DEVICES PERFORMED BY MAGNETIC RESONANCE SAFETY TESTING SERVICES
Testing procedures include those developed by the American Society for Testing and Materials (ASTM) International. Please note, Dr. Shellock serves as a member of the American Society for Testing and Materials (ASTM) International committee that addresses MRI aspects of implants and devices.
A percentage of the proceeds from the projects conducted by
Magnetic Resonance Safety Testing Services
is used to support educational and research activities of the Institute for Magnetic Resonance
Safety, Education, and Research (www.IMRSER.org).
www.MagneticResonanceSafetyTesting.com
MAGNETIC RESONANCE SAFETY TESTING SERVICES
Frank.Shellock@MRIsafety.com.
Specializing in:
COMPANY PROFILE
Frank G. Shellock, Ph.D., the President of MAGNETIC RESONANCE SAFETY TESTING SERVICES has over 30 years of experience as a researcher in the field of magnetic resonance (MR) imaging. He has served as a consultant to many medical manufacturers and has performed MRI testing on more than 5,000 implants and devices.
Dr. Shellock has published more than 240 peer-reviewed articles and the most widely-read medical textbooks on MR safety:
A NEW book is published each year with an updated list of medical implants, devices, and materials that have been evaluated in the MRI environment. This list is consulted by radiologists and MRI technologists World-wide to ensure the safety of patients undergoing MRI procedures.
MAGNETIC RESONANCE SAFETY TESTING SERVICES is uniquely qualified to accomplish all tasks related to the evaluation of medical implants, devices and materials for acceptable use in the magnetic resonance environment.
CONTACT MAGNETIC RESONANCE SAFETY TESTING SERVICES
Visit www.MagneticResonanceSafetyTesting.com
Call (310) 291-6890
or E-mail: Frank.Shellock@MRIsafety.com
for additional information.
© by Shellock R & D Services, Inc. and Frank G. Shellock, Ph.D. All rights reserved.